将0.72g草酸亚铁(FeC2O4)放在一个可称量的敞口容器中高温焙烧,500~600℃时,容器中的固体质量保持0.4g不变。所得物质的化学式为( )
【分析】根据反应前后铁元素的质量不变,确定所得物质中铁元素和氧元素的质量比,进而确定其化学式。
【解答】解:0.72g草酸亚铁中含铁元素的质量为
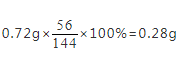
,而反应后固体质量为0.4g,不可能是铁单质,结合选项可知,应为铁的氧化物,铁元素和氧元素的质量比为0.28g:(0.4g﹣0.28g)=7:3,设铁的氧化物的化学式为FexOy,56x:16y=7:3,则x:y=2:3,故所得物质的化学式为Fe2O3。
故选:C。
【点评】本题难度不大,明确反应前后铁元素的质量不变、化学式的有关计算是正确解答本题的关键。
Jack has something important to do this afternoon . so he can’t attend the meeting____.
—How do you study _______ a test?
—I study by working _______ my partner.
—How do you learn English so well?
—________ chatting with my uncle in America on line.
—All of our classmates ______ Jack are going to the welcome party for foreign students on Saturday.
—I guess that’s because he will have to join in the speech contest that day.
He had to sell newspapers seven.
_____ the Hope Project started, it has helped thousands of students return to school.
No one likes a person bad manners.
On April Fool’s Day,people enjoy playing tricks _________ their friends.
The policeman was surprised the news.
E-tickets will be used for high-speed trains ________ the country in 2019. Passengers can enter the station by simply using their ID card.