铜及其化合物在生活、生产中用途广泛。
(1)以氧化铜为原料制取铜。
某研究小组为探究CO的还原性,设计了如下系列实验。
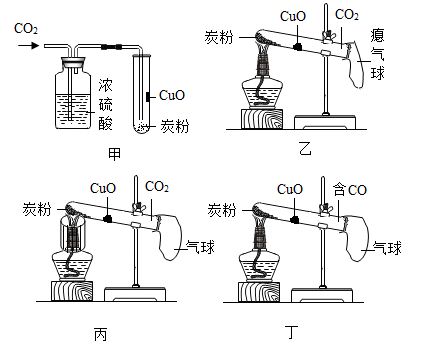
实验1:将CuO加水制成糊状,刷在试管内壁,在试管底部加入炭粉,再向试管中通入CO2(见图甲),集满后立即用气球密封。
实验2:用酒精灯加热CuO部位(见图乙),无明显现象。
实验3:用加网罩的酒精灯加热炭粉(见图丙);一段时间后,利用CO2传感器测得试管内CO2含量变小。
实验4:加热CuO(见图丁),黑色固体变成紫红色。
①实验1中浓硫酸的作用是 ;
②实验2得到的结论是 ;
③实验3的目的是 ,酒精灯加网罩的作用是 ;
④写出实验4中发生反应的化学方程式:。
(2)以辉铜矿(主要成分为Cu2S)为原料冶炼铜。
方法1:火法炼铜.Cu2S在高温下与氧气反应的部分转化关系如图:
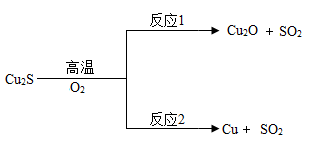
①Cu2O中铜元素的化合价为 ;
②反应2的基本类型是 ;
方法2:生物炼铜。Cu2S在酸性环境和微生物菌类的作用下转化为CuSO4的原理是Cu2S+O2+2H2SO4═2CuSO4+2H2O+X。
③X的化学式为 ;
④从含有CuSO4和H2SO4的混合溶液中回收铜,请补充完整实验方案。
步骤1:向一定量的混合溶液中加入过量的试剂Y,过滤,得到滤渣和滤液1。
步骤2:取步骤1中的滤渣,,过滤,得到Cu和滤液2。
步骤3:将滤液1与滤液2合并,冷却结晶,得到FeSO4•7H2O晶体。
写出步骤1中发生反应的化学方程式:(任写1个)。

Jack has something important to do this afternoon . so he can’t attend the meeting____.
—How do you study _______ a test?
—I study by working _______ my partner.
—How do you learn English so well?
—________ chatting with my uncle in America on line.
—All of our classmates ______ Jack are going to the welcome party for foreign students on Saturday.
—I guess that’s because he will have to join in the speech contest that day.
He had to sell newspapers seven.
_____ the Hope Project started, it has helped thousands of students return to school.
No one likes a person bad manners.
On April Fool’s Day,people enjoy playing tricks _________ their friends.
The policeman was surprised the news.
E-tickets will be used for high-speed trains ________ the country in 2019. Passengers can enter the station by simply using their ID card.